Ganciclovir, a regular medicine used to treat human cytomegalovirus (HCMV) infections, was found less efficient when reacting to human’s NUDT15 enzyme — based on a recent BioMAX study. This finding gives further insight into pharmaceutical treatments’ efficacy in HCMV cases.
Although being regarded as a common disease, HCMV infections might cause severe consequences for immunocompromised patients and those with a history of bone marrow or organ transplants. The infection can cause, among others, sensorineural hearing loss among newborns and CMV syndrome. Today, antiviral agents such as Ganciclovir (GCV) and Valganciclovir are the most common clinical treatments for patients with such infections by targeting the viral DNA polymerase.
The research done at BioMAX, led by Prof. Pål Stenmark from Stockholm University, Sweden, focused on the role of NUDT15 on Ganciclovir’s antiherpes function. The research team found that human NUDT15 enzyme hydrolyzes the active metabolites in the medicine and therefore limit its efficacy for such antiviral treatments. “This has implications on how the medicine is used, such as the dose that would be effective for treatment,” explained Stenmark.
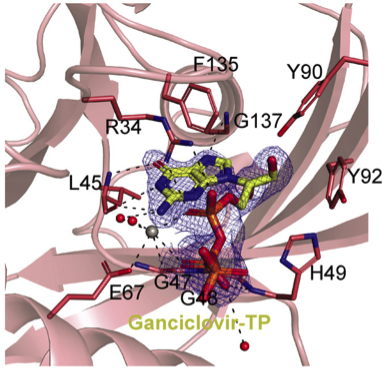
Biochemical analyses was used to bring forward the data on the study, while its binding modality is observed through NUDT15/GCV-TP co-crystal structure.
Getting high-quality structures of NUDT15-Ganciclovir complexes was the major challenge faced by the team of researchers of the study. “It was challenging to get a good NUDT15-Ganciclovir complex. Many crystals were tested, and a very high concentration of ganciclovir triphosphate had to be used to obtain a high-quality structure,” explained Stenmark.
Digging deeper into NUDT15
Using MAX IV’s brilliant X-rays, researchers have done various studies on NUDT15 enzymes. Especially its role in pharmaceutical drug metabolization. In previous studies, partly done at MAX IV (3, 5), researchers found that NUDT15 metabolizes a group of anticancer medicines (Thiopurines). In this follow-up study, researchers found that Ganciclovir, one of the most common antiviral medicine, is metabolized by the NUDT15 enzymes.
“The data from MAX IV have made it possible to see how inhibitors and substrates bind to human NUDT15 enzymes with atomic accuracy,” explained Stenmark.
Although the knowledge of NUDT15 enzymes and their effects on pharmaceutical drugs are acquired, their natural function is still unknown. The group of researchers would like to dig deeper into this.
“The natural function of the enzyme is something we would like to explore further (6),” explained Stenmark.
References
References 1-6 are based on close collaborations between Prof. Stenmark (SU/LU) and Prof. Jun Yang (St. Jude Children’s Research Hospital) or Prof. Thomas Helleday (KI).
- Zhang SM, et al. (2021) NUDT15-mediated hydrolysis limits the efficacy of anti-HCMV drug ganciclovir. Cell Chem Biol.
- Nishii R, et al. (2021) NUDT15 polymorphism influences the metabolism and therapeutic effects of acyclovir and Ganciclovir. Nat Commun 12(1):4181.
- Rehling D, et al. (2021) Crystal structures of NUDT15 variants enabled by a potent inhibitor reveal the structural basis for thiopurine sensitivity. J Biol Chem 296:100568.
- Valerie NC, et al. (2016) NUDT15 Hydrolyzes 6-Thio-DeoxyGTP to Mediate the Anticancer Efficacy of 6-Thioguanine. Cancer Res 76(18):5501-5511.
- Suiter CC, et al. (2020) Massively parallel variant characterization identifies NUDT15 alleles associated with thiopurine toxicity. Proc Natl Acad Sci U S A 117(10):5394-5401.
- Carter M, et al. (2015) Crystal structure, biochemical and cellular activities demonstrate separate functions of MTH1 and MTH2. Nature Commun 6:7871.



