High-capacity energy storage systems are an important part of the renewable energy transition and can be realised using RTILs, room temperature ionic liquids, as electrolytes. A research team from University of Tartu, Estonia, recently used beamline FlexPES to study the stability of RTILs for such applications.
Energy can be stored by separating negatively and positively charged atoms or molecules, ions, of a substance, called an electrolyte. Liquid electrolytes are found in, for example, batteries or supercapacitors. Salts contain negative and positive ions and, for example, table salt, sodium chloride, contains positive sodium ions and negative chloride ions but has a melting point around 800 °C. RTILs are salts that are liquid already at room temperature.
Safer and more effective
RTILs are an attractive choice of liquid electrolytes because they are a safe, non-flammable, non-volatile and not requiring any added solvent. They also increase the amount of electrical energy stored in a capacitor by letting the device operate without damage over a larger voltage range than with conventional electrolytes. There are several types of RTILs, and experiments are needed to study their properties in detail in different device configurations and physical conditions.
X-rays show function in detail
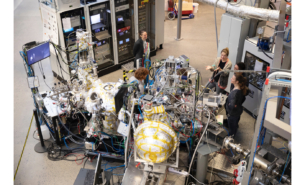
The researchers are using the light from MAX IV to investigate the interaction of the RTILs with various electrode materials and voltages to find the limits of the operation without damaging the device.
“This gives to us information about the electrochemical stability of the investigated RTIL and electrode material,” says Dr. Jaanus Kruusma from University of Tartu.
The investigations are done with the electrochemically polarised electrodes soaked in the RTIL electrolyte located in an open vessel located directly in the XPS analysis chamber. The RTIL does not evaporate in the high vacuum as their vapour pressure is very low at room temperature which is an advantage for the experiments. The XPS measurements are done in situ, meaning that this device is working in similar to real conditions.
“Our results are useful for those who are designing new or improving existing high capacity and power electrochemical energy storing systems such as supercapacitors and electrochemical cells,” says Dr. Jaanus Kruusma.
More to RTILs than energy storage
RTILs have other applications than energy storage. They are also used as the electrolytes in solar energy to electrical energy converting cells, in organic synthesis as the medium and co-catalysts, as lubricants and corrosion inhibitors. Next level would be to perform the measurements at near atmospheric pressure conditions to investigate the properties of liquid systems having a more moderate vapor pressure.
“There are still very little investigations already done in this field and very little systematic information published. Exciting results come without warning so systematic work must be done for years,” says Dr. Jaanus Kruusma.