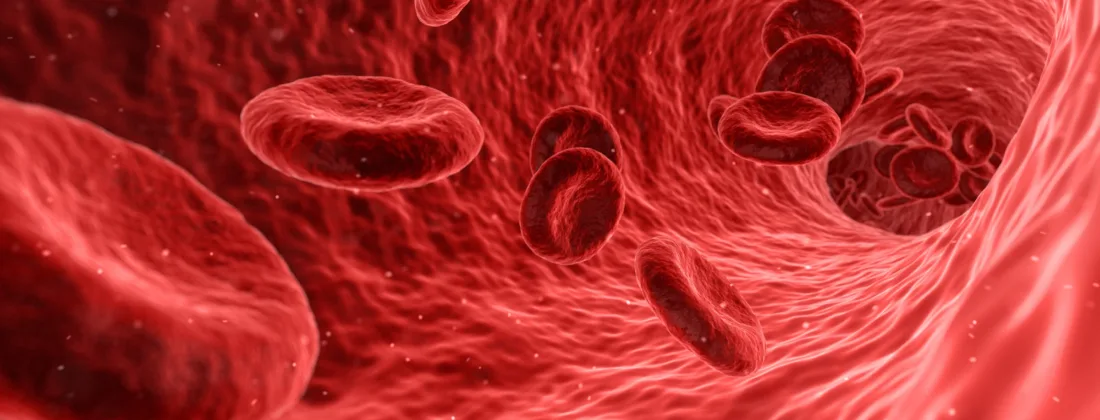
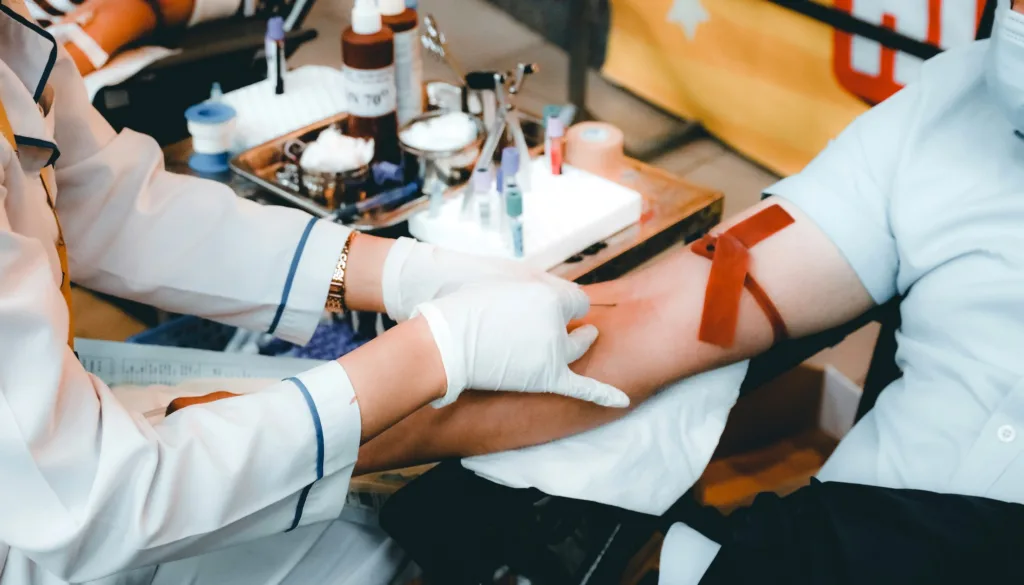
In clinical practice it is well established that type O blood, which lacks A and B antigens on the red blood cells, can be safely used in universal blood transfusions for any ABO blood group. Serious or even fatal immune reactions may occur if one receives incompatible blood from a donor. How might we mitigate the risks for low donor supply or unusable blood in emergencies? Research groups from the Technical University of Denmark (DTU) and Lund University now report in Nature Microbiology, an enzymatic conversion method to create ABO-universal blood, a major leap towards human blood that could potentially enable live-saving blood donations to anyone, without negative immune response or the need for matched donor-recipient blood types. Data for the structural determination of key enzymes used in conversion of the ABO-universal blood was collected at MAX IV’s BioMAX beamline.
The researchers investigated a previously unexplored aspect of blood compatibility with enzymatically converted (ECO) blood. “What is different is that we targeted extensions of the A and B antigens. Our work has uniquely addressed these extensions, using the specialist human gut symbiont Akkermansia muciniphila,” said Maher Abou Hachem, co-lead scientist of the study, and Professor in the Department of Biotechnology and Biomedicine at DTU. “This inspiration is a result of an informal meeting in the Nordic Glycobiology Seminar, where two PhD students met and learned about the discovery of the group B extended antigen by co-lead scientist and Professor of transfusion medicine Martin L. Olsson and his group at Lund University, and the work of my group on the mucin utilisation strategy of Akkermansia muciniphila.”
There is tremendous progress in the crystallographic field. Now that a single person easily can determine and analyse 2-4 structures it has become a physical approach which is a natural part of every engineering project. — Jens Preben Morth
Enzymatic conversion of blood type B was first demonstrated in 1982, with promising initial experiments using an α-galactosidase to cleave B antigens from coffee beans. Lund University Professor Olsson and Professor Henrik Clausen at Copenhagen University later discovered bacterial enzymes able to cleave A and B antigens from the surface of red blood cells (RBC). The latest development is an efficient two-step removal of the A-antigen from Stephen Withers’ laboratory at the University of British Columbia. The type of antigens (terminal motifs on sugar chains) on the cell surface determines an individual’s blood type, A, B, AB, or O, and the compatibility between blood donors and recipients.
Discovery crystallised
The research groups investigated carbohydrate-active enzyme (CAZy) families with known activities and expressed their enzymes. After tests on model oligosaccharides, a panel of A. muciniphila enzymes was selected based on their excellent mucin-degrading properties, assayed, and further optimised for cleavage of RBC antigens. Their work produced two enzyme blends for RBC conversion: one for blood group A antigens and one for group B antigens, including their carbohydrate extensions.
With X-ray diffraction measurements at BioMAX, the group has analysed six of the 22 enzymes to date, according to DTU Biotechnology Professor and study researcher Jens Preben Morth. “While some of these enzymes have been relatively easy to crystallise, others have been recalcitrant, but the substantial crystallisation efforts resulted in a large bounty as they revealed induced fit dynamics which are crucial for understanding the details of enzyme mode of action, in addition to revealing previously unknown carbohydrate binding motifs in novel carbohydrate binding domain that will define a new family.”
“The determination of enzyme structures in different states and bound with carbohydrate substrates, products, or substrate analogues brought novel insight into the molecular recognition and dynamics of the enzymes,” explained Abou Hachem, adding that detection of the new carbohydrate binding family would have been impossible relying on AlphaFold modelling alone.
The promising study is de facto work in progress. The notable efficiency of the new enzymatic cocktails from Akkermansia muciniphila vary based on the type of antigen tested. “Conversion of group B blood has so far been the most successful, while group A antigens are more complex,” explained Martin L. Olsson. “Even so, our new enzyme mix displays significantly better compatibility with group O plasmas than if only the regular A is clipped off, but work is underway to understand and improve remaining incompatibility of the converted cells.”
The group is currently investigating structural and mechanistic details of the interactions with red blood cells, with good expectations for more structural detail. The earlier work of this study was funded by Research Fund Denmark, Technology and Production Sciences, whereas the current work is funded by the Novo Nordic Foundation.