Carbon dioxide, emitted mainly by combustion of fossil fuels, is harmful to the climate and the main reason for increased global warming. Diverting carbon dioxide into hydrogen carriers or chemicals such as methanol, a valuable raw material and energy carrier, is thus highly desired. Supported metal nanoparticle heterogeneous catalysts such as copper on zinc oxide is used for the catalytic conversion of carbon dioxide to methanol. Researchers have now discovered that it is possible to avoid by-products and at the same time make the process more sustainable by adding a small amount of gold to the catalyst.
Carbon dioxide can be converted into methanol and water by reaction with hydrogen. The reaction is only possible in the presence of a catalytic material such as Au or Cu nanoparticles supported on zinc oxide. The chemical reaction will then take place on the particle surfaces. In a recent study, a research team from Germany, Japan and Sweden have shown that modifying the typical ZnO-supported Cu nanoparticles by a small amount of gold (< 10 weight percent) makes the reaction more selective.
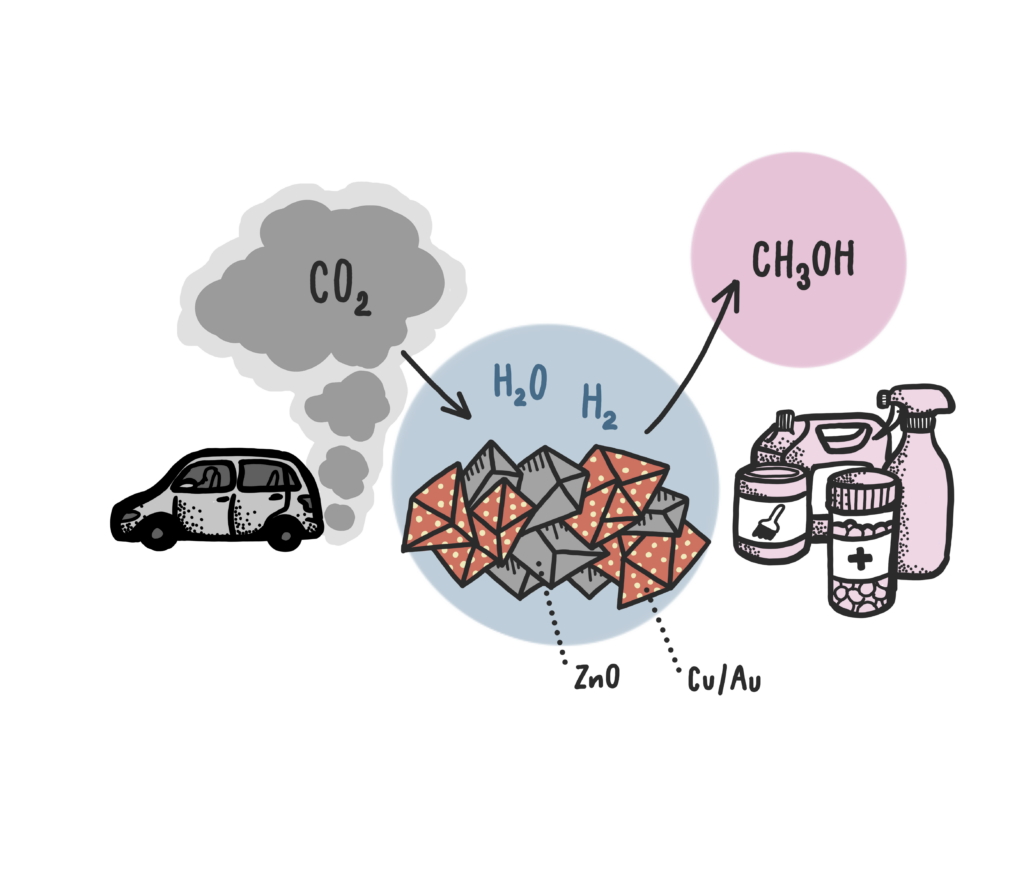
“We saw that the selectivity of methanol increased up to 100% at rather low temperature without the formation of undesired by-products such as carbon monoxide,” says Dr. Ali Abdel-Mageed, one of the study’s authors and the PI of the project.
The catalytic process has also often been relying on solvents that are problematic from a sustainability perspective. With the added Au, the reaction can be carried out in water. Water is not only a green solvent, but it can also be used as a medium for the capture of carbon dioxide from the air.
“The next step is to develop the process so that the conversion of carbon dioxide can be made at lower temperatures and with higher reaction yields. It would be even better from an energy and a process safety perspective,” says Dr. Abdel-Mageed.
The researchers have used MAX IV to study how the conversion of carbon dioxide works at a detailed level with the added gold. The method used was Ambient Pressure X-ray Photoelectron Spectroscopy (AP-XPS) at beamline SPECIES. AP-XPS allows for surfaces to be investigated under a realistic atmosphere and pressure, which is important for dynamics.




