Photoelectron spectroscopy (PES) is based on the photoelectric effect, the fact that matter irradiated by photons of sufficiently high energy emits electrons. Information about the sample is determined from the intensity, the angular distribution, and the spin of the emitted electrons. An additional attribute of electrons is that they can only travel a very short path in a solid before they collide with an atom and lose energy. This means that only electrons from the very top layers of a solid sample will emerge without energy loss, and energy-resolved electron spectroscopy is therefore a standard tool in surface science.
The kinetic-energy measurement of the electrons is often done by deflection in an electric field, most commonly in hemispherical mirror analyzers (as at both permanent endstations at FlexPES), or by measurement of the time of flight of the electrons (as can be done with the mobile ICE endstation). Here is a sketch depicting typical photoelectron spectroscopy experiment setup with a hemispherical analyzer:
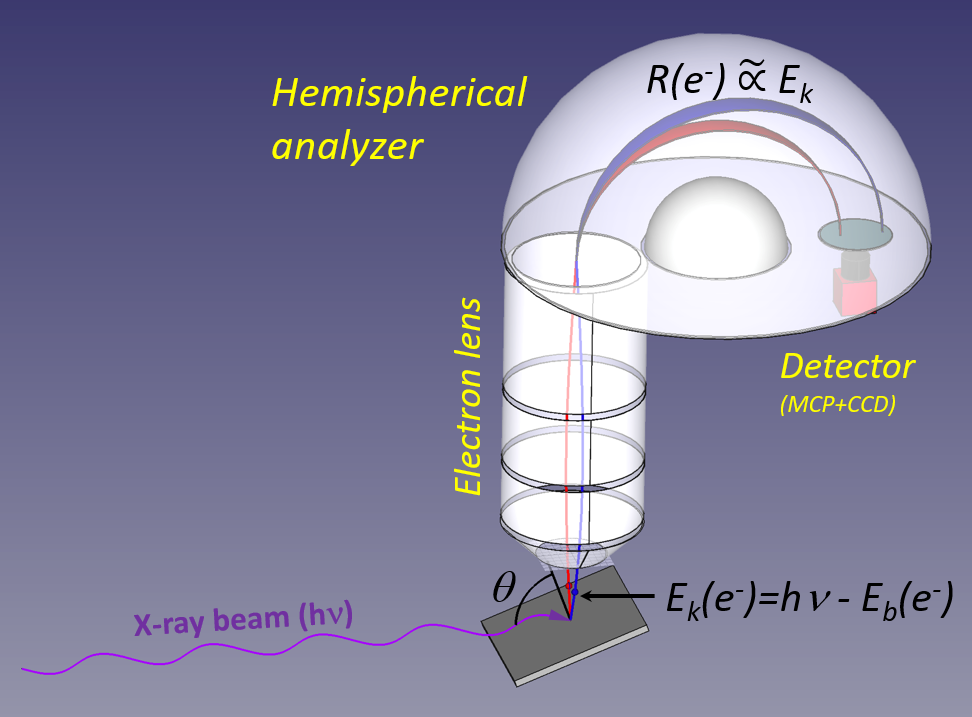
X-ray photons with a well-defined energy (hν) impinge on a sample, and the emitted electrons have a kinetic energy given by the difference between the photon energy and the electron binding energy. At the FlexPES beamline, the photon energy range is between 40-1500 eV, and there are provisions allowing a wide range of experimental situations for solid, liquid, or gas-phase samples. The emitted electrons are collected and transported by an electron lens to the hemispherical analyzer, where the electrons are deflected by the electrostatic field between two charged hemispheres. The radius of curvature of the electron trajectory depends (nearly linearly) on the kinetic energy, and in the figure the red and blue-colored trajectories are for electrons with lower and higher kinetic energies, respectively. After traveling through the hemisphere, the electron is detected with a position sensitive detector (often a micro-channel plate in combination with a phosphor screen + fast camera or any other read-out).
Photoelectron Spectroscopy is useful in order to gain insight regarding:
- The abundance and/or chemical state of a particular element in a sample
- Charge transfer processes
- Nuclear motion (ex. ultra-fast dissociation)
- The mechanical, electrical and magnetic properties of a condensed matter sample
The PES techniques that are possible using the FlexPES hemispherical analyzer setups include:
- Core-level photoelectron spectroscopy
- Auger electron spectroscopy
- Resonant Photoemission/Resonant Auger Spectroscopy (RPES/RAES)
- Angle-Resolved Photoelectron Spectroscopy (ARPES)
- X-Ray Photoelectron Diffraction (XPD)
Core-level photoelectron spectroscopy
Perhaps the most straightforward application of PES is core-level photoelectron spectroscopy, which involves the study of the deeper lying electron levels. The energies of the core levels are distinct for each element, which makes it easy to distinguish between for instance nitrogen and oxygen. The core levels do not contribute to the bonding in matter, but they are indirectly influenced by the chemical surroundings in such a way that the binding energy is changed, and this is referred to as a “chemical shift”. Core-level PES is therefore often used to investigate the abundance and chemical state of a particular element in the sample, for instance the oxidation state of a catalyst, or the protonation state of an acid or a base.
Auger Electron Spectroscopy (AES)
After the core ionization event, the atom is in an unstable electron configuration, and decays rapidly to a lower energetic state. This can happen via the emission of an X-ray photon, or by emission of an electron, a so-called Auger decay, and this is the most common process for light elements and shallow core levels. In the same way as core-level photoelectrons, Auger electrons are sensitive to the chemical surrounding of the ionized atom, but the spectra are often more difficult to interpret (due to overlap of many possible final states with open-shell character). An advantage of Auger electron spectroscopy is that it is independent of the excitation, meaning that the resolution in the electron spectrum does not depend on the photon energy bandwidth.
Resonant Photoemission/Resonant Auger Spectroscopy (RPES, RAES)
In conventional photoelectron spectroscopy, the electron is ejected in an almost instantaneous process, using a photon energy that is higher than the threshold of ionization. By tuning the photon energy, one can instead excite a core electron to an unoccupied level near the threshold. This excited state, with a hole in the core level, will rapidly decay by emission of an electron, just as in the Auger decay after a core photoionization event, or a photon. The decay is fast, of the order of a few femtoseconds or faster, but some dynamic processes occur on the same time scale, e.g. charge transfer processes or nuclear motion of light atoms, and the electron spectra recorded using resonant excitation can thus give insight into such processes. The resonant process selects a particular element or even a particular chemical state, which means that the information will also be particular to the situation close to the selected atom. Since electron emission is induced by resonant photoexcitation, and the process is similar to an Auger decay filling a core hole, it is referred to as both Resonant Photoemission and as Resonant Auger Spectroscopy. This method has been used to; for instance, investigate how quickly charge moves in a light-harvesting molecule in a solar cell, or how the dissociation speed of a molecule is affected by condensation.
Angle-Resolved Photoelectron Spectroscopy (ARPES)
In condensed matter, the valence electron orbitals overlap and form bands. By studying the photoelectron emission at different angles, it is possible to investigate the distribution of electrons of these bands (in the so-called reciprocal space). From this technique, often called band mapping, information about mechanical, electrical, and magnetic properties of the sample can be obtained. The Surface&Material Science (SMS) branch of the FlexPES beamline offers (limited) possibilities to perform ARPES experiments (see “Endstations” for more details).
X-ray Photoelectron Diffraction (XPD)
Electron diffraction is based on the wave character of electrons – just like light (electromagnetic waves), electron waves can be scattered and result in interference patterns. This technique is commonly used as a means to study crystal structure in connection to electron microscopy (SEM and TEM). The electrons emitted in a photoionization event will also be diffracted, and by utilizing this effect, it is possible to at the same time obtain structural information from the diffraction pattern, and chemical information from the spectrum.