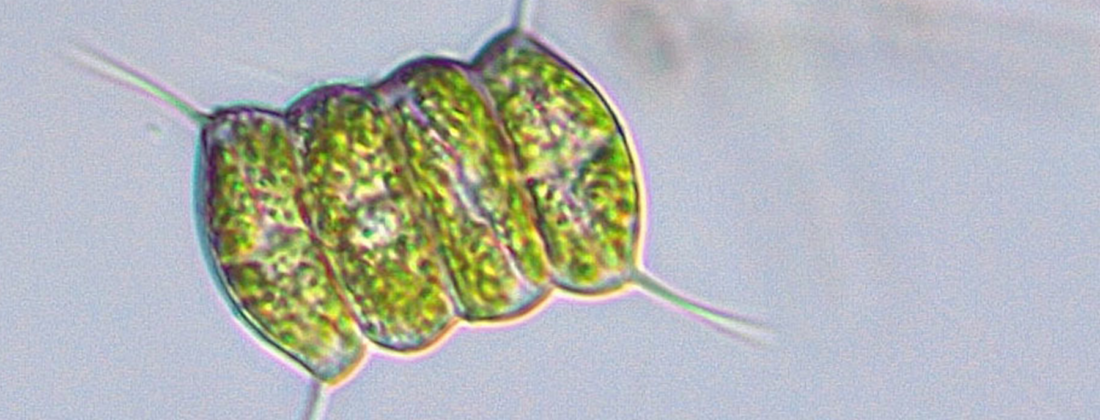
Microalgae for pollution removal is the topic of two recent studies by MAX IV users. The storage mechanism of phosphorous in the algae was investigated in detail contributing to method development for pollution removal from wastewater. The phosphorous-containing algae can, in turn, be used to soak up metal pollutants.
Phosphorous is used as a fertiliser to enhance crop yields in agriculture. It is needed to feed a growing population but can also become a pollutant if uncontrolled. Agriculture and wastewater treatment processes are the primary sources of phosphate pollution and eutrophication, causing oxygen depletion and loss of aquatic life.
It is known that microalgae can take up and store phosphorous from water sources. In recent studies, researchers have investigated the storage process in more detail to optimise it and find uses for the algae after they have done their cleaning. Imaging with X-rays revealed the granules that form when microalgae store phosphorous.
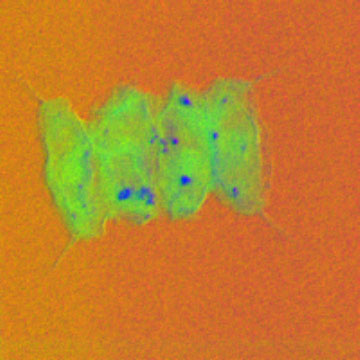
“We found that the granules are composed of an interesting polyphosphate compound, inositol hexaphosphate, also known as phytic acid. This compound is found in plant seeds such as grains, nuts or pulses. It is interesting that algae can also store phosphate [editors note: a chemical compound of phosphorous] in this form. The storage is triggered by first starving the algae of phosphate and then giving them a surplus,” says Prof Richard Haverkamp from Massey University in New Zealand, one of the researchers behind the study.
The phytic acid has further uses for pollution removal, so the microalgae seem to come with a bonus.
“Because phytic acid is known to react with some metal ions, we can use the existing knowledge about phytic acid reactions with metals to predict which metal ions might be able to be absorbed readily by these algae containing phosphate granules. We have just started investigating this as a way to clean up water polluted with these metals or to remove valuable metals from aqueous sources,” prof Haverkamp continues.
The researchers scanned the whole microalgae through the X-ray beam to measure the phosphate content using a method called Scanning Transmission X-ray Microscopy (STXM).
“STXM has the ability to provide images with elemental and chemical information on the features present in the image. So whereas in transmission electron microscopy it is possible to see an object that we can label a granule, in STXM we can image that object but can also measure that it contains phosphorus and that this phosphorus is a specific compound of phosphorus,” says Prof Haverkamp.
The researchers saw the phosphate-rich granules with better than 60 nanometres resolution. They complemented the analysis by studying a sample where the microalgae cells were mixed together to investigate the form of phosphorous compounds in larger detail. However, phytic acid was the only phosphorus compound found. They studied two different kinds of algae.
“We wanted to test whether the concepts we were studying were likely to be general, not just specific to one species. Therefore, having more than one species in the study helps us to see if this might be so. We chose two species of algae that come from different orders to have significant biological differences,” explains prof Haverkamp.
In the second study of one of the algal species, the focus was on possibilities for taking up the type of metals called rare earths.
“The next step was that we wondered if these algae with phosphate granules would be good traps for rare earth elements in solution, where the rare earth elements might combine with the phosphate granules. Of the two rare earths we studied, cerium and gadolinium, only the gadolinium combined with the phosphate granules. While that means we can’t easily remove both rare earths, this could be an advantage – it might be a good method to separate mixed solutions of rare earths. They are normally found together, so they have to be separated, which can be difficult because of their closely similar chemistry,” says prof Haverkamp.
The research team are already investigating the algae further.
“We are also interested in whether these phosphate granule-containing algae might be useful in removing undesirable metal pollutants from rivers or wastewater sources. We have a study underway to test this,” Prof Haverkamp concludes.
Photo (top): Petr Knotek/Botanicalrealm.com (under CC BY)